-
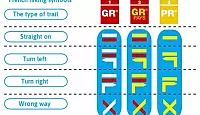
GR, GRP, PR: What do the French hiking signs mean?
What are the coloured symbols on French hiking routes? Who paints them there and why?
-
Miss France: glam - but not sexy
Miss France organiser Geneviève de Fontenay fears she is fighting a losing battle to protect her 'Cinderella dream' from vulgarity
-
Normandy Landings visit for Queen
Queen Elizabeth has confirmed a state visit to France, ending rumours she is handing over duties to Charles
Euro health change after PIP scandal
New health safety rules introduced in wake of French manufactured breast implant scandal
NEW European rules on the safety of medical devices have been introduced in the wake of the worldwide scare over faulty PIP breast implants from France.
The new rules cover the 80-odd "notified bodies" in the European Union that are responsible for the inspection of 10,000 medical devices - from plasters to pacemakers.
"We now have a clearer basis for unannounced audits, sample testing, or joint assessments by notified bodies," said the Commissioner for Consumer Policy, Neven Mimica.
The new rules states that a member state can only designate a notified body after a "joint assessment" involving experts from the Commission and other nations. Authorities also will be required to regularly monitor notified bodies.
The inspection bodies for their part will have to randomly carry out unannounced factory audits and check for the substitution or adulteration of raw-materials.
A total of 16,426 women have had breast implants removed since it was found in 2011 that Poly Implant Prothese (PIP), manufactured in La Seyne-sur-Mer in the Var, were twice as likely to rupture as rival brands, and that the manufacturer used industrial silicone to fill them.
PIP founder Jean-Claude Mas, 73, has been charged with manslaughter and fraud. PIP's implants have been banned and the company has been liquidated.
Previous articles:
Breast implant woman dies
Faulty implants should be removed
Breast implant bosses arrested
Photo: AFP Photo - Anne-Christine Poujoulat
© AFP/Connexion