-
Festive travel reminder for residency card holders as new border checks expand in France
The EU’s Entry/Exit System is being rolled out progressively
-
Road blockades by farmers continue on Monday despite call for Christmas truce
A meeting between local union leaders and officials in Toulouse will determine pre-Christmas blockades
-
Retired French butcher takes Chronopost to court over year-long missing parcel
Parcel containing homemade foie gras went missing in December 2024 but butcher says company did not acknowledge issue
French lab: Our treatment can help with Omicron but awaits approval
The Nantes research centre says many other antibiotics currently prescribed in hospitals are not as effective to the new variant
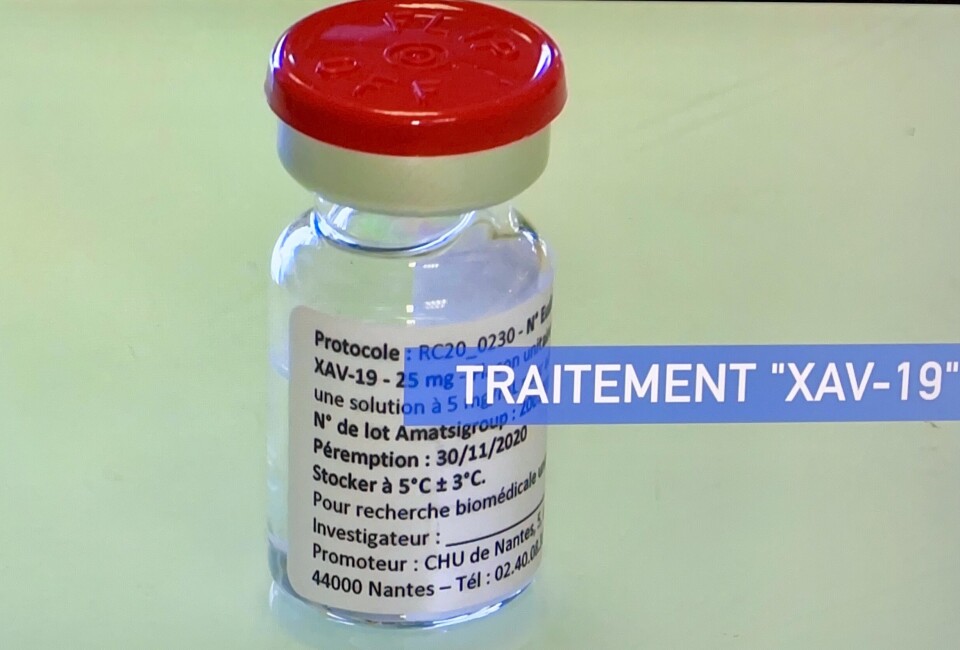
A medical research laboratory in Nantes says tests show its Covid-19 treatment, awaiting final authorisation for use, is effective against the Omicron variant unlike many other antibiotics currently prescribed in hospitals.
The head of Xenothera, Odile Duvaux is appealing to the government to give their new medicine, XAV-19 the green light as soon as possible:
“For over a year, we have been insisting that vaccinations and treatments are both needed in the fight against the epidemic.
“We hope that the 25,000 doses of XAV-19 which have already been made and are ready for use will be able to benefit those patients who need it as soon as possible.”
The treatment is aimed at patients who are developing respiratory problems and would be used to prevent them getting any worse and going into intensive care.
It uses polyclonal antibodies which are efficient at fighting complex viruses, and laboratory tests show it has the capacity to block Omicron. The laboratory says that in contrast, the usual monoclonal antibodies are showing themselves less efficient against the new variant.
The laboratory is waiting for the final results from the clinical trials and authorisation from the ANSM Agence nationale de sécurité du médicament and the Haute autorité de santé, and hopes it will be on the market by the beginning of 2022. The government has pre-ordered 30,000 doses.
Read more: France orders 30,000 doses of Nantes lab's Xav-19 Covid treatment
A year ago, Odile Duvaux told The Connexion she hoped XAV-19 would be on the market by June 2021, but she said analysis of the data had taken much longer than anticipated.
“It is frustrating, but above all sad, it is taking so long, because it could have already benefitted so many patients who are ill.”
She said the laboratory could have been the first in the world to have had an antibody treatment ready as they had already been working on previous coronavirus outbreaks and by March 2020, they had prepared a treatment for earlier coronavirus strains.
However, vaccinations were continuously prioritised over treatments and she says that for a long time she felt she was a lone voice insisting on the necessity of developing treatments:
“No-one really listened to what I had to say. All we heard from the politicians was vaccination, vaccination, vaccination. I am not at all against vaccinations, but we need both.
Read more: ‘Our Covid-19 treatment was ready but red tape stopped us’
“Treatments will always be necessary. However good a vaccination it will only work for 95% of the population, so there will always be a proportion who will get ill. That could be around 4 million people in France, and some of those will require a treatment like ours. We have estimated that XAV-19 would benefit about 50,000 patients in France a year.”
Xav-19 is one of several other treatments which have recently been approved by some government health authorities or are awaiting final approval. Pfizer has an oral antiviral treatment, Paxlovid; Merck has Molnupiravir, there is Ronapreve from Regeneron-Roche and Regkirona from Celltrion in South Korea.
Mrs Duvaux says they are a tiny lab finding itself in competition with some of the world’s largest companies:

“Even so there is a place for us on the market as our treatment is not the same as any other and destined for a different group of patients. Some treatments are for immediate use for anyone with a high risk factor of getting a serious form of the illness such as those with heart conditions. Ours is for any patient who has come to hospital because they are beginning to develop secondary symptoms.
“As well as interest from France, we have continual requests for our treatment from countries all over the world.”
She says it has been a new experience working in the same domain as the world’s biggest players:
“It has made me sad to see that though there are very many people who have good intentions, there are also high stakes which mean it is not always very “clean”.
There are conflicts of interest which exist and I have observed that the outcome is not always in the best interest of patients. I find that distressing as I have an idealistic side to me.”
Though she says it is frustrating it is taking too long to get their treatment onto the market, which could have saved lives, she says over the past year the process has been speeded up:
“Normally it would take around three years for the clinical trials plus approval from the ANSM. We had our first contact with the ANSM in April 2020 and a year later we were recruiting the last patients for our first clinical trials.
“That is something unheard of in normal times. In the last few months the final results of the tests are being processed at the same time as the authorisation procedure, and usually the two do not happen in parallel.”
Related articles
France announces 11 new anti-Covid measures
More cases, fewer deaths: How France’s 5th Covid wave compares so far
























