-
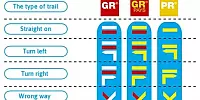
GR, GRP, PR: What do the French hiking signs mean?
What are the coloured symbols on French hiking routes? Who paints them there and why?
-
Miss France: glam - but not sexy
Miss France organiser Geneviève de Fontenay fears she is fighting a losing battle to protect her 'Cinderella dream' from vulgarity
-
Normandy Landings visit for Queen
Queen Elizabeth has confirmed a state visit to France, ending rumours she is handing over duties to Charles
Shake-up in drug safety laws
Appetite suppressant Mediator 'should have been banned 10 years ago', says watchdog
THE SYSTEM by which medicines are approved for use will be reformed in the wake of the scandal over diabetes drug Mediator.
The medicine, prescribed in France from 1976-2009, is suspected of having caused 500-2,000 deaths and to have caused heart damage to thousands of other users. A report by social affairs inspection body IGAS has said it should have been banned at least 10 year ago.
Health Minister Xavier Bertrand said that, while the report indicated the makers of the drug, Laboratoires Servier, carried much of the blame, the state's system of checks on medicines' safety had "failed its mission" and "serious weaknesses" had been flagged up.
He said he wanted a new, more "transparent", system including having patients' representatives and ones from independent medical press (such as Préscrire, which opposed Mediator for several years) on commissions that approve drugs.
Afssaps, the body charged with evaluating their safety, should be fully state-funded and not, as now, by taxes on drug companies, he added.
Mr Bertrand said new drugs should be shown to be more effective than existing ones, not just better than placebos. Drugs should also be suspended from the market "more more quickly and easily" where there are safety concerns.
"If there is doubt, then it must always work in the patient's favour," he said.
La Mutualité Française, representing many top-up health insurance policies, has also called for rapid change, and a law is expected to be presented to parliament before the end of the year.
The IGAS report's accusations against Servier include allegations that it knew that Mediator was a powerful appetite supressant, a kind of product subject to strict restrictions in France, but changed the name of its main ingredient so it was not recognisable as such.
The firm's founder, Jacques Servier, 88, is to appear in court this month to answer charges brought by victims.
Following the IGAS report, the firm issued a statement saying it was "amazed" by the responsibilities it appeared to place on them, "which do not appear to accord with reality".
CISS, a group of health sector associations, is asking the government to set up a victims' compensation fund.