-
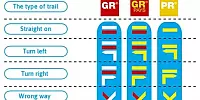
GR, GRP, PR: What do the French hiking signs mean?
What are the coloured symbols on French hiking routes? Who paints them there and why?
-
Miss France: glam - but not sexy
Miss France organiser Geneviève de Fontenay fears she is fighting a losing battle to protect her 'Cinderella dream' from vulgarity
-
Normandy Landings visit for Queen
Queen Elizabeth has confirmed a state visit to France, ending rumours she is handing over duties to Charles
Watchdog monitors 59 daily medicines
Painkillers, asthma inhalers and anti-smoking drug are on watchdog’s alert list for possible dangerous side-effects
DRUGS watchdog Affsaps has published a list of 59 drugs it has on special alert in response to the furore over the diabetic drug Mediator.
The wide-ranging list includes an asthma drug, a morning-after pill, an osteoporosis drug and a pill to help smokers quit.
Health minister Xavier Bertrand has promised wide-ranging reforms in the wake of the revelations over Mediator, which was widely used as a slimming pill but is feared to have caused the deaths of at least 500, and possibly as many as 2,000, people in France.
However, the “risk control” list is being monitored by the drug manufacturers themselves, a process called into question in the Mediator scandal and which prompted one third of French people to say they have no confidence in the drug-monitoring regime.
The Mediator scandal was revealed by health magazine Prescrire. Director Bruno Toussaint told Le Parisien of the new list: “Certain of the drugs should be pulled off the market, others have some use, but we do not know if the monitoring is effective.
“At the moment, the manufacturers’ laboratories are monitoring the side-effects and then telling Affssaps: it would be better to have independent experts.”
He later named the anti-inflammatory drug Arcoxia and tobacco control drug Champix as medicines that should be withdrawn. Arcoxia’s side-effects include possible brain haemorrhage and heart defects, while Champix is said to cause depression, irritability and headaches.
Mr Bertrand has told Affssaps to speed up checks on the medicines and give him a report in two weeks. He has called for the agency to have sufficient funding to ensure its independence of the drug firms it is monitoring, and for doctors to declare any links to pharmaceutical companies.
Affssaps has published the list of 59 medicines on its website. Patients taking these drugs should continue to take the drug and contact their doctor:
Abstral, Aclasta 5mg, Acomplia, Alli 60mg, Antasol, Arcoxia, Byetta, Celsentri, Cervarix, Champix, Chlorhydrate de Buprenorphine, Cimzia, Cymbalta, Effentora, Efient 10mg, Ellaone, Entonox, Exjade, Firmagon, Galvus, Gardasil, Ilaris, Increlix, Instanyl, Intelence, Intrinsa, Isentress, Januvia, Kalinox, Kuvan, Lucentis, Methadone AP-HP gellule, Multaq, Mycamine, Nplate, Onglyza, Orencia, Oxynox, Pradaxa, Prevenar 13, Procoralan, Relistor, Revlimid, Revolade, Roactemra, Stelara, Symbicort, Thalidomide, Thelin, Toctino, Tracleer, Tysabri, Tyverb, Valdoxan, Vimpat, Volibris, Xarelto, Xyrem, Zypadhera