-
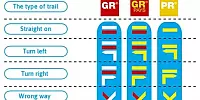
GR, GRP, PR: What do the French hiking signs mean?
What are the coloured symbols on French hiking routes? Who paints them there and why?
-
Miss France: glam - but not sexy
Miss France organiser Geneviève de Fontenay fears she is fighting a losing battle to protect her 'Cinderella dream' from vulgarity
-
Normandy Landings visit for Queen
Queen Elizabeth has confirmed a state visit to France, ending rumours she is handing over duties to Charles
Call for drug ban over death fears
French medical journal calls for anti-vomiting drug to be withdrawn after it was linked to a number of deaths in 2012
A FRENCH medical journal has called for a widely prescribed anti-vomiting drug to be withdrawn, claiming it may have contributed to between 25 and 120 deaths in France in 2012 alone.
The drug, domperidone, which has been used to treat nausea and vomiting since the 1980s and is sold under the brand Motilium, as well as more than a dozen generics, has been linked to cardiac problems.
Now, French medical journal Prescrire has said that the drug’s effectiveness is "too modest to justify the exposure to premature deaths."
The call comes as the European Medicines Agency is set to publish the results of a year-long study into the effects of domperidone. This is due for publication in March.
That study was launched after medical authorities in Belgium raised concerns about the drug.
According to medical insurance data, about three million people in France were prescribed the drug in 2012. Prescrire estimated that up to 23 million French people have had at least one prescription for domperidone between 2003 and 2013.
The journal noted that studies by Canadian and Dutch teams have shown an increase in the frequency of sudden death in people exposed to domperidone.
In 2011, the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM) flagged up the increased risk of sudden death associated with domperidone. It also warned against the unauthorised use of Motilium to ease breastfeeding.