-
Shop donations: Pays de la Loire and Corsica are France’s most generous regions
Almost €15 million was raised in 2025 via ‘round up’ donations at checkouts
-
Train passengers in Occitanie to be reimbursed over frequent delays and cancellations
See eligibility conditions and find out how refunds will be issued
-
Marine Le Pen appeal: Prosecutors call for election ban to be upheld
The court of appeal will return a decision before the summer, ahead of the 2027 elections
France could approve Pfizer vaccine for under 12s by end of this year
Covid vaccine producers Pfizer/BioNTech have published results of its trials showing their vaccine is ‘safe’ for children aged 5-11, and are seeking approval to use it in Europe
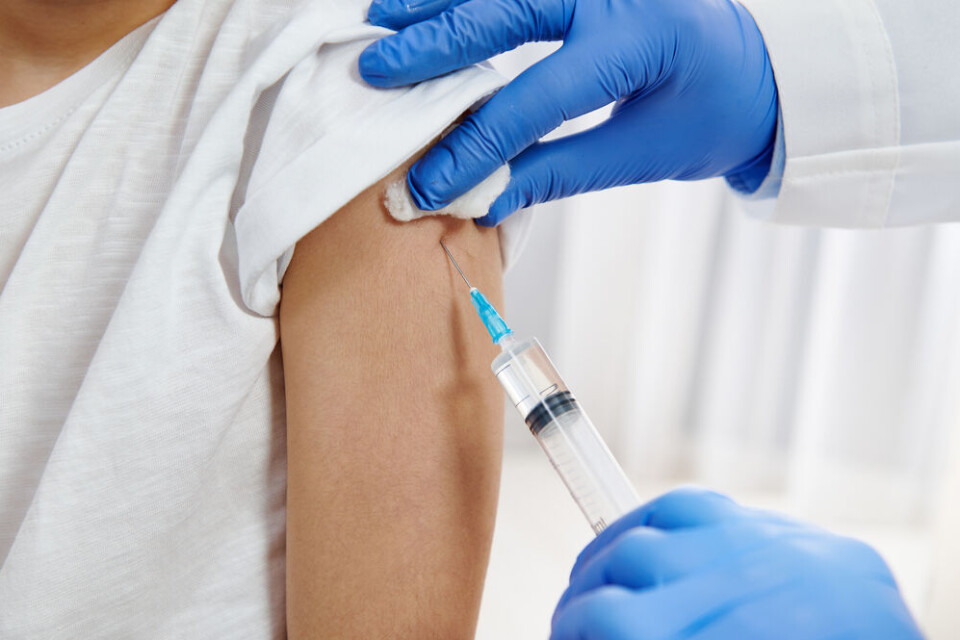
This week, the Pfizer/BioNTech laboratories confirmed that their Covid vaccine is "safe" for children aged five to 11. It is now seeking use approval from the European Medicines Agency.
Its findings were based on trials conducted on 4,500 children of up to 11 years old in the US, Finland, Poland and Spain.
Pfizer and BioNTech intend to apply to the health authority the European Medicines Agency (EMA) for approval to administer their vaccine to children under age 11 in Europe "as soon as possible".
Dr. Ugur Sahin, CEO and co-founder of BioNTech, said in the report: “We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season.”
“The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When could under 12s be vaccinated in France?
Approval in the EU (including France) could be granted by the end of the year, towards the end of November.
This will depend on the time that elapses between the laboratories publishing their results and approval being granted by the European Medicines Agency, as well as the Haute Autorité de Santé in France.
What are the current age range rules in France?
For now, only children and teenagers over 12 years of age can receive the Covid vaccine, the Pfizer/BioNTech vaccine having been approved for the 12 to 17 age bracket in mid-June and the Moderna approved in July.
Take up has been driven in part by the decision to extend the health pass scheme to under 18s from October, which could prevent some from accessing venues like restaurants and cinemas.
The government currently remains focused on vaccinating as many teenagers as possible. Back in the summer it said it had no plans to vaccinate the under 12s.
What are other countries doing for this age group?
Most countries in the world are not currently vaccinating children aged under 12. Cuba is the only one to have authorised Covid vaccines for children as young as two.
In the UK, the chief medical officers have recommended that healthy 12-15 year olds are offered one dose of a Covid vaccine in an effort to minimise disruption to their education.
However, “the health benefits of universal vaccination in children and young people below the age of 18 years do not outweigh the potential risks” the Joint Committee on Vaccination and Immunisation (JCVI) in the UK has so far said.
The JCVI “advises that children and young people aged 12 years and over with specific underlying health conditions that put them at risk of serious COVID-19, should be offered COVID-19 vaccination” only.
Yet, it was reported yesterday that the Pfizer vaccine could be approved for children aged 5 to 11 as soon as next month in the US.
Related articles
Covid France: More research needed before under 12s are vaccinated
























