-
La Voie Bleue: European Cycle Route of the Year is in France
700km bike path linking Luxembourg and Lyon has been crowned winner of the 2026 title
-
MAP: See how your location in France affects online food shop prices
New analysis shows how your shop compares on average
-
Further sightings of processionary caterpillars in France prompt action from local authorities
Caterpillars have arrived early after mild winter
Five Covid test and vaccine updates for France this week
Change to PCR rules, the end of the 15-minute waiting time after boosters, new school protocol, the arrival of the Novavax vaccine and more…
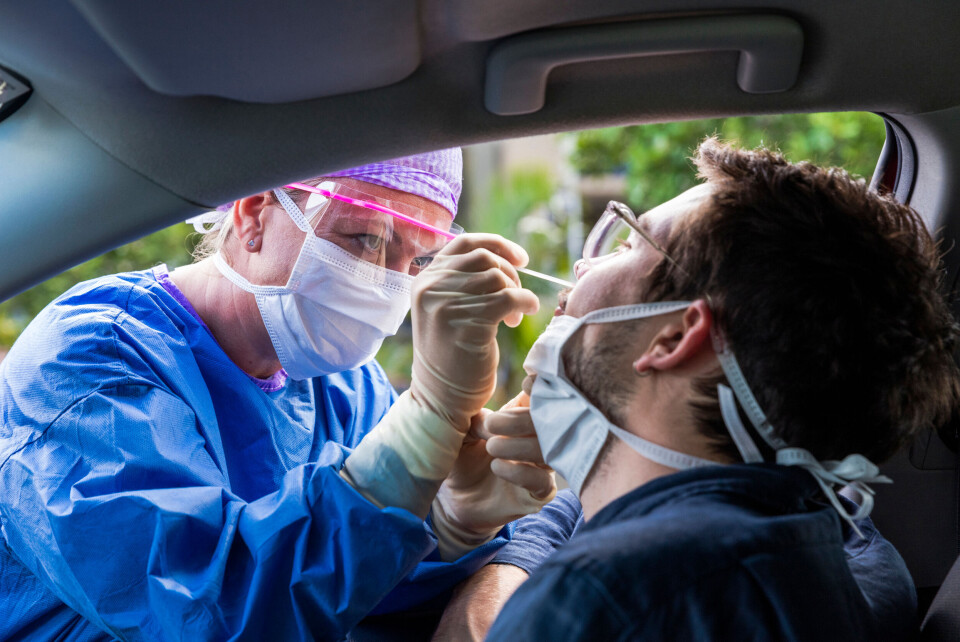
We look at the Covid-related updates which have taken place around France this week.
1. Confirmatory PCR no longer necessary after positive lateral flow
People in France who receive a positive antigen test result in a test carried out by a health worker no longer need to carry out a confirmatory PCR test.
However, you must still take a PCR test if you carry out a self-administered test and it returns a positive result, as these tests can often produce errors if not used correctly.
Read more:Covid France: PCR no longer needed after positive antigen test
A confirmatory PCR in this case will also enable people testing positive to enter their result on the Health Ministry’s SI-DEP platform and to later obtain a recovery certificate to bypass unneeded vaccine doses in the months that follow.
2. End of the 15-minute wait after a booster dose
People will not need to wait at the vaccination centre for 15 minutes after receiving their booster doses, the health ministry has announced.
People who have just been injected with a vaccine dose are normally advised to stay at centres for this length of time, just in case they have an adverse reaction to the jab.
However, if these people did not suffer an allergic reaction after the first two injections, the risk is “extremely low” when they go for their booster, the ministry said.
The change, which came following advice published by the Conseil d’orientation de la stratégie vaccinale, “will help to fluidify the organisation of vaccination centres in the context of booster campaigns.
“Among the notable drug safety information surrounding Covid vaccinations that has been recorded on a national level, the Agence nationale de sécurité du médicament et des produits de santé [drug safety authority] has not been notified of a concerning case of serious undesirable effects in the 15 minutes following vaccination,” the ministry added.
People will still need to wait for 15 minutes after their first and second vaccine doses, and if they are injected with a vaccine other than Pfizer or Moderna, if they are pregnant, or if they have a known allergy or are at risk of anaphylactic shock.
3. Both parents’ consent needed for vaccination of five to 11-year-old
Permission from two parents or guardians and not just one will now be required for the vaccination of a five to 11-year-old, it has been announced.
Only one parent or guardian needs to be present at the appointment but they must come with a sworn statement confirming that the other is in agreement.
Children aged 12 to 15 need consent from one parent only.
By January 4, 67,000 five to 11-year-olds had received at least one vaccine dose. These children can now also receive their second injection as early as 18 days after their first, although the 21-day gap remains the optimal time frame.
4. Covid testing rules relaxed in schools
France’s new Covid testing rules for schools have been eased slightly just after they were introduced.
Since January 3, each time a positive Covid case has been detected in a class, primary school and vaccinated secondary school pupils have needed to take a PCR or antigen test, and then two self-administered tests on day two and day four, and restart this process with each new case.
Now, if a new positive case emerges in the week that follows a first case, pupils will not need to restart this three-test cycle, which will remain valid for seven days.
Unvaccinated children over 12 have to self-isolate for seven days on the detection of a Covid case in their class.
5. France could see first Novavax injections in February
The first infections of the US’s Novavax vaccine could take place in France at the beginning of February.
The government is awaiting advice from healthcare quality regulator, the Haute Autorité de Santé, which is expected in the coming days.
Novavax is the fifth Covid vaccine to be approved by the European Medicines Agency. Unlike Pfizer and Moderna, it is not a mRNA vaccine but is a more traditional subunit vaccine.
This means that it uses antigenic parts of the pathogen – but not all of it – to elicit an immune response.
This method is less ‘revolutionary’ than the technology used in mRNA vaccines, and so may assuage the concerns of people who are concerned about the ‘experimental’ nature of the vaccines currently available.
Clinical trials have suggested that Novavax is 90% effective against Covid, and offers near full immunity against serious illness with the virus. It also offers protection from the Omicron variant.
Related articles
False passes, Covid cases, hospitalisations: Latest figures for France
Coronavirus: Daily updates on the situation in France
France and UK ease travel rules as Omicron spreads in both countries
























