-
French farmers begin major protest in Strasbourg
More than 700 tractors expected to park outside EU Parliament in two-day protest
-
French Prime Minister confirms use of Article 49.3 to pass budget
Usage opens government up to motion of no confidence but opposition MPs unlikely to find enough support
-
Floor collapses in Paris building leaving 20 injured
Water leaking into the building from balcony is thought to be cause of collapse
French health authority approves vaccination of five to 11-year-olds
Vaccination should, however, remain voluntary for 5.4 million children in this age group, says the Haute Autorité de Santé
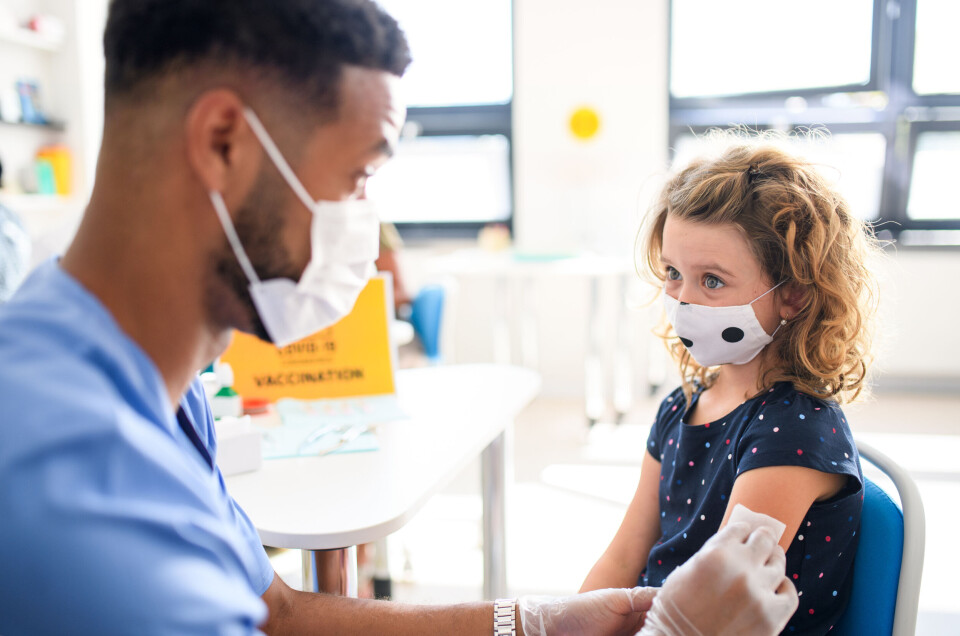
France’s health service quality regulator Haute Autorité de Santé (HAS) has today (December 20) approved the extension of the vaccination campaign to all children aged five to 11.
Children in this age group who are classed as being at higher risk from Covid have been able to get vaccinated since December 15.
The HAS’ decision follows that of the Comité consultatif national d’éthique (CCNE), which approved the use of the Pfizer vaccine on younger children last week.
Both the HAS and the CCNE have stipulated that vaccination should remain voluntary for this age group, and that younger children should not be made subject to health pass requirements.
“The HAS proposes that the vaccination programme be opened to children [aged five to 11], without obligation and without their vaccination status conditioning the acquisition of a health pass,” the health authority wrote in a statement adding that the campaign should prioritise children under 12 who are already at collège.
“Severe forms of Covid rarely affect children but, when this is the case, nearly 80% of these cases are found in children who do not already have serious health conditions,” the HAS added in justification of its decision to extend the campaign to all children.
It continued that in the context of the Omicron variant, a rise in the number of serious cases occurring among children could be expected, making vaccination more important.
Younger children will be injected with two doses of the Pfizer vaccine at an interval of three weeks, but using a dosage which is three times smaller than that given to adults.
The HAS states that this adapted vaccine “displays a very high efficacy against the dominant variants which are currently circulating and its capacity to prevent serious illness is excellent.”
The benefit-risk ratio has therefore been deemed to be in favour of vaccinating children by the European Medicines Agency and the US Food and Drug Administration.
This latest extension of the vaccination campaign could concern up to 5.4 million children.
However, the HAS has recommended that vaccination only be carried out following a blood test to determine whether the child in question has had Covid in the past. If they have, then they will only receive one vaccine dose.
The HAS reached its conclusion on child vaccination after examining the findings of clinical studies carried out by Pfizer, as well as drug safety data from around the world, modelling of the impact of vaccination on child infections and the advice of the Comité d’éthique.
The government is also waiting for approval from the Comité d’orientation pour la stratégie vaccinale, which should be delivered on Wednesday morning (December 22).
If this body is in favour of child vaccinations, the first doses could be administered on Wednesday afternoon.
The government has stated previously that it hoped to offer the vaccine to all children in this age by the end of the year.
Related stories
Vaccinating children is 'a necessity’ says French PM
Hospitals in France ‘paralysed’ by non-vaccinated, says leading medic
Health advisors call for New Year Covid restrictions in France
























