-
UK’s ETA transition period ends: what changes for residents of France?
Rules have been toughened for those with dual UK or Irish and another citizenship
-
Quotas for wolf shootings relaxed after rise in attacks in France
Farming unions say the changes do not go far enough
-
28.3C: new February temperature record set in south-west France
Records broken across the country but risk of late frosts remains
France approves three vaccines adapted to Covid Omicron subvariants
The vaccines – which are technically not ‘new’, but adaptations of the existing formulas – will be prioritised for at-risk people in a campaign this autumn
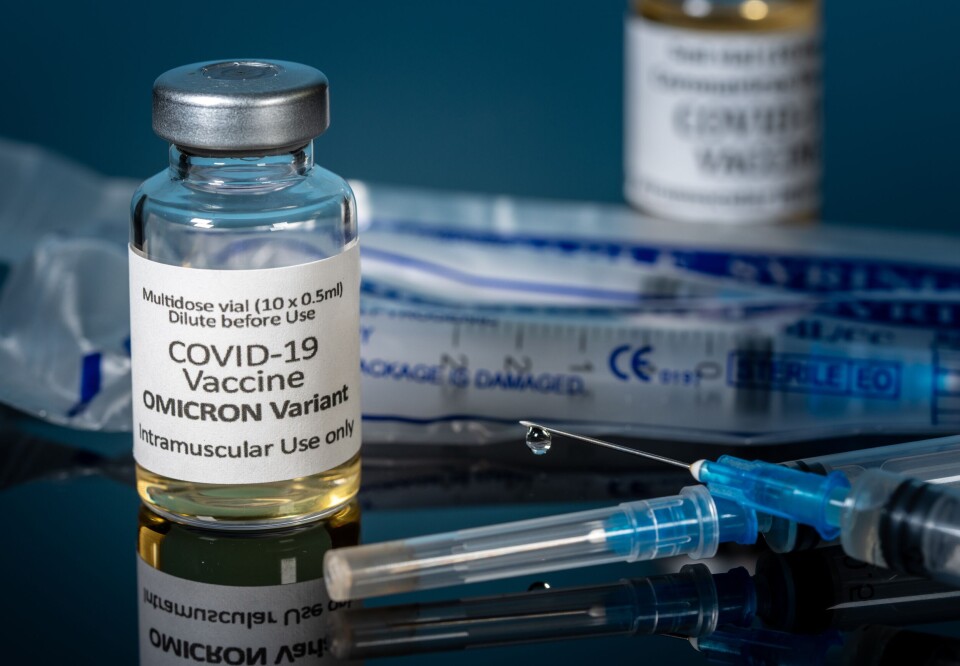
France has approved three ‘new’ vaccines designed to target the Omicron variant of Covid-19, ahead of a new vaccination campaign this autumn.
The Haute autorité de santé (HAS) gave its authorisation on Wednesday, September 21. The vaccines are set to be used in a new vaccination round, alongside the usual winter flu vaccine campaign.
The HAS said that its decision had been made “based on available data within an epidemic context, marked by the spread of the subvariant BA.5”.
They are “not ‘new’ vaccines per se, but [existing ones] that have been adapted to [current] circulating strains”, said the HAS. “The clinical effectiveness of these new vaccines is at least equivalent, if not higher [and] their tolerance is identical.”.
The vaccines will be prioritised for people who are at the highest risk of a severe case, as well as their carers and close contacts.
These groups include people aged 60 or over, and people under 60 who are at high risk, including people with underlying conditions, immunosuppressed people, children and teenagers at high risk, and pregnant women. Their immediate contacts will also be eligible.
Two of the vaccines have been created by Pfizer/BioNTech, and the third by Moderna. All use the mRNA technology (which triggers an immune response), and are adapted to fight the BA.4 and BA.5 sub-variants of the Omicron strain.
They were recently validated by the European Medicines Agency.
❗️ La HAS autorise les vaccins Pfizer et Moderna adaptés à #Omicron BA.1 ou BA.5.
— Nicolas Berrod (@nicolasberrod) September 20, 2022
Ils pourront être utilisés "indifféremment" en rappel pour les personnes à risques, une fois qu'ils seront disponibles en France ces prochaines semaines. @le_Parisien
1/14https://t.co/UyKXOPLUjB pic.twitter.com/EvarzGx7v7
The ‘new’ vaccines are recommended regardless of the vaccine that was originally given.
The vaccines come as France looks set to experience a new, eighth wave of Covid this winter. The Omicron strain has been dominant throughout 2022, but increased cases have been reported in recent weeks.
Read more: ‘Eighth wave of Covid imminent in France’ says health ministry
The BA.4 and BA.5 strains have been responsible for new waves in Europe and the US recently.
On Tuesday (September 20), the health ministry said: “The Ministry of Health and Prevention will take note of the opinion of the HAS, as it has since the beginning of the health crisis, and will decide on the vaccination strategy to be implemented on the basis of the recommendations.”
It has now confirmed that a new vaccination campaign, alongside the usual flu drive, is set to begin on October 18. The idea is to offer people both vaccines on the same day, or close together, if possible.
The HAS added: "For the time being, and because the number of cases of infection has been on the rise again in recent days, those most at risk are advised not to wait to receive their second booster, if they have not yet had it.”
The authority said that despite recommendations, only about 30% of people over 60 have received a second booster dose, which is needed to offer full protection.
The original versions of the vaccines are still effective against a severe version of the disease, the HAS said, and they are still the only ones that can be used for a first vaccination.
Related articles
Eighth Covid wave in France this autumn expected to be less severe
Leading epidemiologist warns of new Covid wave in France this summer
























