-
La Voie Bleue: European Cycle Route of the Year is in France
700km bike path linking Luxembourg and Lyon has been crowned winner of the 2026 title
-
Before and after: Garonne river floods in south-west France
Satellite images show extent of flooding from back-to-back storms in February
-
Home insurance increases expected in France after floods
Compensation costs for the recent storms and flooding across the west and south-west is estimated to be in the billions of euros
Malta bars UK tourists given Indian-made vaccine: Will France do same?
The Mediterranean island is the first EU country to rule on the issue of batches made at Serum Institute of India – a site not recognised by the European Medicines Agency as a maker of the official AstraZeneca jab
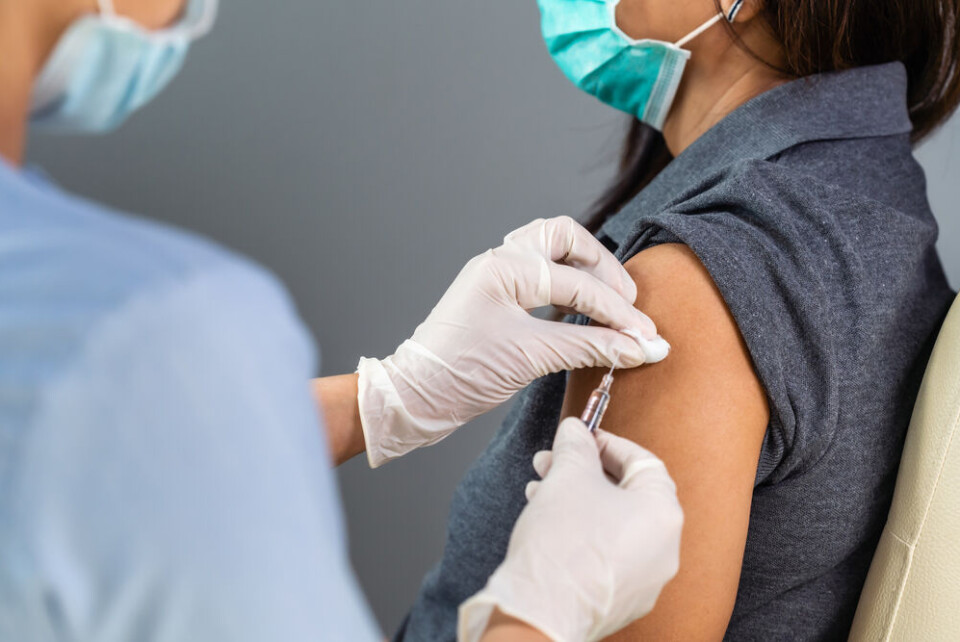
Visitors from the UK vaccinated with an Indian-made version of the AstraZeneca vaccine identified as such by batch numbers are not being accepted into Malta, raising the question of whether other countries will follow.
Malta requires all arrivals from the UK to provide proof of full vaccination and it is no longer accepting people vaccinated with batch numbers which identify the vaccine as one of the batches the UK received that were made by the Serum Institute of India (SII).
It is the first EU country to have taken an explicit decision on this point, according to The Connexion research – however, so far, there is no indication that France will follow suit.
The UK is known to have received some five million doses of the vaccine earlier this year, with batch numbers 4120Z001, 4120Z002 and 4120Z003.
What is France’s view on this Indian-made vaccine?
Many EU countries, including France, state they do not accept the validity of vaccinations with ‘Covishield’, the brand name for the vaccine made by SSI in an agreement with AstraZeneca.
This is based on the fact that the European Medicines Authority (EMA) has given no specific marketing authorisation for this vaccine nor, significantly, has it to date approved the Indian factory site so as to add its vaccine to the authorisation for AstraZeneca.
An application for the latter has been made by AstraZeneca and AstraZeneca states it expects a decision “shortly”.
The UK government and medicines authority have said that the batches the UK received from SSI did not have the name ‘Covishield’, but only AstraZeneca, however SSI makes only one vaccine substance in its partnership with AstraZeneca.
As a result, vaccination certificates, whether paper or digital, from the UK say ‘AstraZeneca’ or ‘Vaxzevria’ (AstraZeneca’s commercial name for its jab), not ‘Covishield’ and are not identifiable at a glance as different from other vaccinations with AstraZeneca.
The UK medicines’ authority (MHRA) considers the batches it received to be identical in nature to other AstraZeneca doses, and there has been no indication that this is not the case or that they are any less effective than those made at EMA-approved sites.
France’s Health Ministry confirmed to The Connexion it does not recognise Covishield and will not do so until it is approved by the European Medicines Authority (EMA) but the ministry has not replied to our queries as to whether border officials will be asked to check batch numbers.
We have heard no reports of people with UK-administred AstraZeneca vaccines being refused travel or entry to France.
None of the major transport companies we have asked has confirmed checking for batch numbers for travellers from the UK.
A spokesman for ferry firm DFDS said: “We will be checking that passengers have full vaccination status as requested by UK and French authorities but operators will not be checking which specific vaccine or batch that customers have received, only that the NHS App or NHS certificate is complete.
“My understanding from discussion with DfT is that all Astra-Zeneca vaccines administered in the UK are Vaxzevria as that is the only vaccine authorised for use, and the NHS does not administer Covishield.”
Evidence of vaccination with an EMA-approved vaccine is required by France for all visitors from the UK unless they give an essential reason for their trip, not including a holiday or spending time at a second home.
It comes as there are reports in the UK media of a couple being turned away at Manchester Airport for a flight to Malta, due to having one of the Indian-made vaccine batch numbers. According to The Telegraph the decision was made by employees of the Tui travel agency.
The Connexion has asked for a comment from Tui. A Manchester Airport spokesman said: “This isn’t something we’d comment on formally but we would, of course, always encourage passengers to check on the Covid restrictions in place at their destination before travelling.”
The EMA has told The Connexion that the only version of AstraZeneca it currently approves is the ordinary one, now known as Vaxzevria. It added that for a vaccine to be considered as such for EMA authorisation purposes it must be made at a site it has checked, which is not the case for SSI.
The UK’s transport minister, Grant Shapps, told BBC Breakfast he would be taking the issue up with the Maltese authorities.
He said: “It is not right and it shouldn’t be happening. The medicines agency, the MHRA, have been very clear that it doesn’t matter whether the AstraZeneca you have is made here or the Serum Institute in India, it is absolutely the same product, it provides exactly the same levels of protection from the virus.
“So we will certainly speak to our Maltese colleagues to point all this out. Obviously it is up to them what they do. But we will be making the scientific point in the strongest possible terms. There is no difference, we don’t recognise any difference.”
Read more
When will Covishield vaccine be accepted under France’s travel rules
























