-
Shop donations: Pays de la Loire and Corsica are France’s most generous regions
Almost €15 million was raised in 2025 via ‘round up’ donations at checkouts
-
Train passengers in Occitanie to be reimbursed over frequent delays and cancellations
See eligibility conditions and find out how refunds will be issued
-
Marine Le Pen appeal: Prosecutors call for election ban to be upheld
The court of appeal will return a decision before the summer, ahead of the 2027 elections
More than 16,300 people in France affected by pacemaker fault
The US manufacturer alerted the medical agency regarding the issues. Here are the pacemakers affected
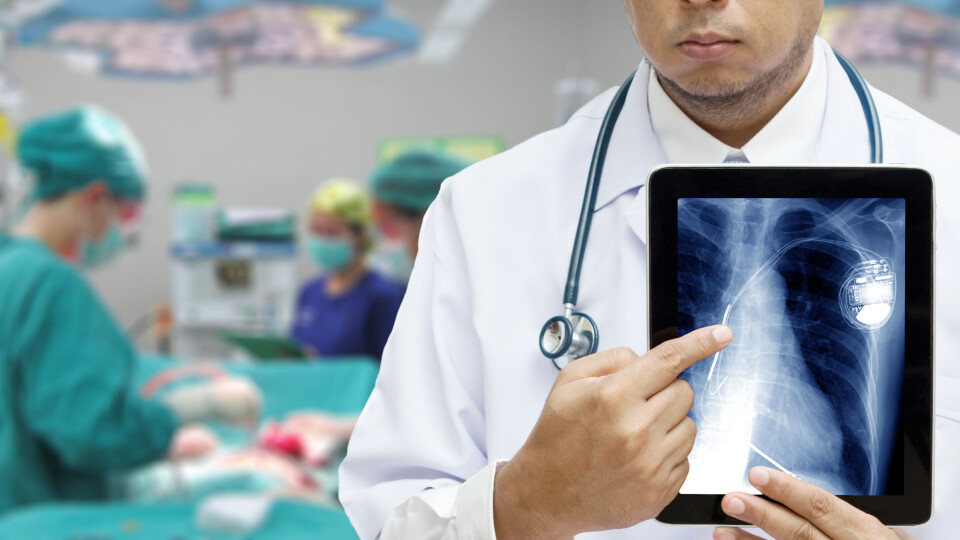
More than 16,300 people in France are thought to be affected by a fault in pacemakers from the brand Abbott, with patients set to be contacted by health establishments to let them know the next steps.
Medical safety agency l'Agence nationale de sécurité du médicament et des produits de santé (ANSM) issued the alert after the US manufacturer reported the problem to them.
The double-chamber pacemakers from Abbott-St. Jude Medical have a fault in their seal.
ANSM said that “there is very little risk of them suddenly ceasing to function… but defective pacemakers may reduce heart stimulation, go into emergency mode, or the battery life may be shortened”.
“The patients concerned will be contacted by the health establishments that installed the device. A cardiologist will then decide whether the device should be removed,” it said.
The defect applies to pacemakers made and distributed between September 2019 to April 2022.
Around 50 pacemakers have already been reported to the health authorities. The manufacturer has told ANSM that those already reported had been fitted in the past 18 months.
The models affected are:
-
Assurity range, serial numbers of model PM2272
-
Endurity range, serial numbers of model PM2172
Patients fitted before September 1, 2019 with Abbott-St. Jude’s Medical models (Assurity and Endurity), or with another band of pacemaker, are not affected by the fault, the ANSM said.
























